Aims
The overall aim of the Project is to combine innovative technologies into a clinical US system that will lead to a radical change in the way fetal screening is performed. At the very least we would like to substantially improve the diagnostic accuracy of scans carried out by less expert sonographers, potentially leading to a screening programme that does not depend on expert sonographers during the initial routine scans.
Aim 1: Improved US acquisition through advanced Multi-Input Multi-Output (MIMO) & Robotics
Multi-probe System
- Work with Philips as an industrial collaborator to develop a multi-probe prototype fetal imaging system.
- Develop robotic systems to hold and position the probes which will allow the probes to be:
- Positioned appropriately for individual patients and fetal positions
- Adjusted during examinations for optimal data acquisition
- Key requirements for the robotic system will include safety, comfort and ease of workflow.
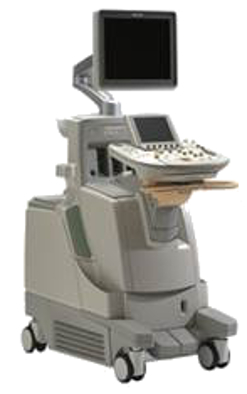
Figure 1: Multiple transducer ultrasound system being developed in Philips Research
MIMO System
MIMO communication systems use multiple antennae in the transmitter and receiver to improve the performance of a device (e.g. an ultrasound probe) without needing to increase the bandwidth or power required. Such MIMO systems have already been developed in other disciplines such as mobile phone networks. We will:
- Explore a fully integrated MIMO concept for ultrasound imaging.
- If successful we will integrate the MIMO system into the Philips prototype.
Aim 2: Develop comprehensive 3D fetal MRI to support information extraction from ultrasound
Our researchers have pioneered Slice to Volume Reconstruction (SVR) methods which allow detailed 3D MRI of the fetal brain with unparalleled anatomical contrast and resolution. These can be used to create detailed fetal brain atlases and allow automatic labelling of key features.
- Further develop the SVR approach to provide comprehensive 3D MRI of all major fetal organ systems and the placenta.
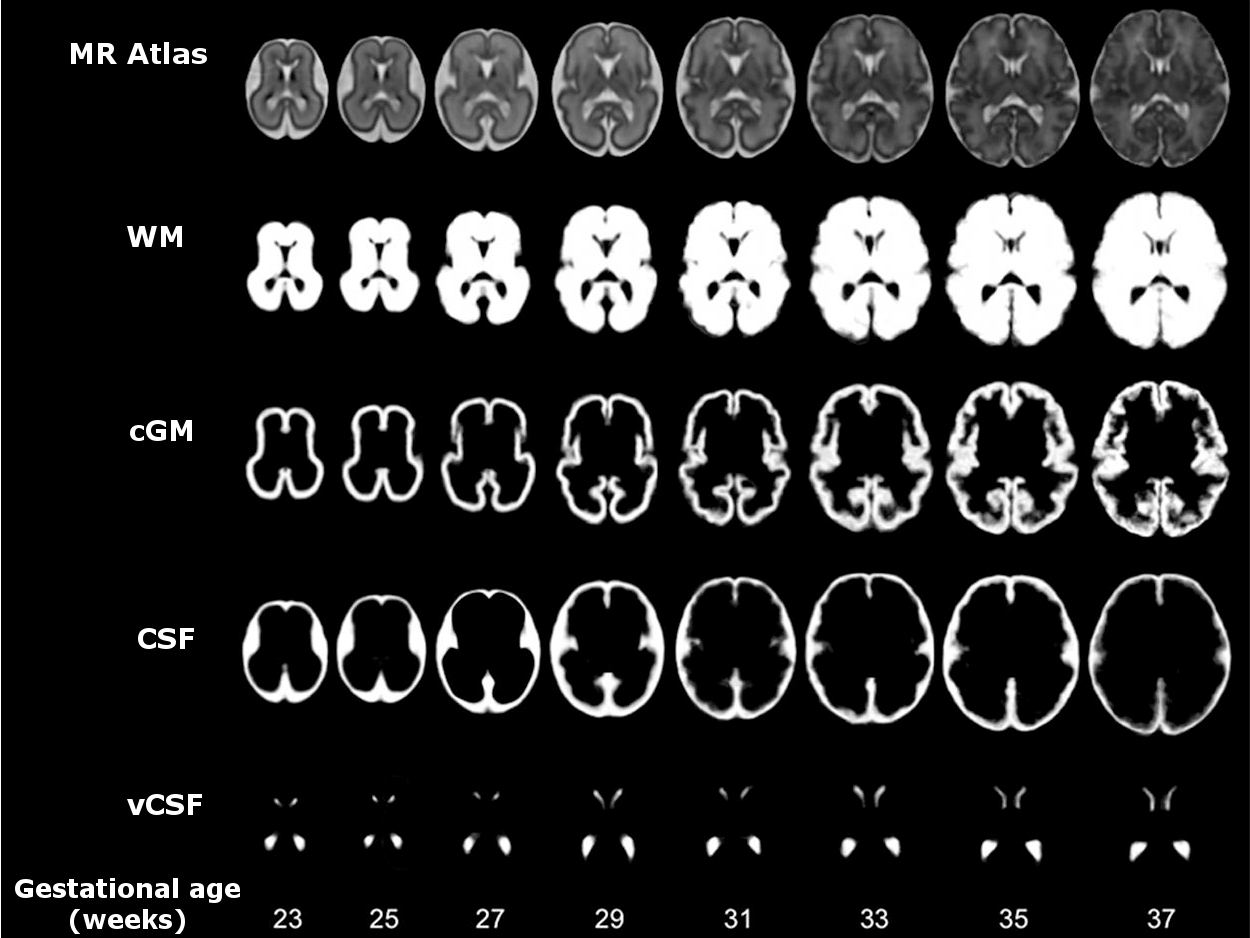
Figure 2: Spatio-temporal atlas of the fetal brain constructed from MRI between 23 and 37 weeks gestational age. The top row shows the MR atlas and the rows below show the tissue probability maps for White matter (WM), Cortical grey matter (CGM), Cerebrospinal fluid (CSF) and Ventricular CSF (vCSF).
Aim 3: Automatic extraction of comprehensive, rich and clinically useful information from ultrasound data
Fetal Atlases
- Create highly detailed annotated joint MRI-Ultrasound atlases of fetal anatomy
- Use the atlases to label and navigate through the new ultrasound data as it is acquired
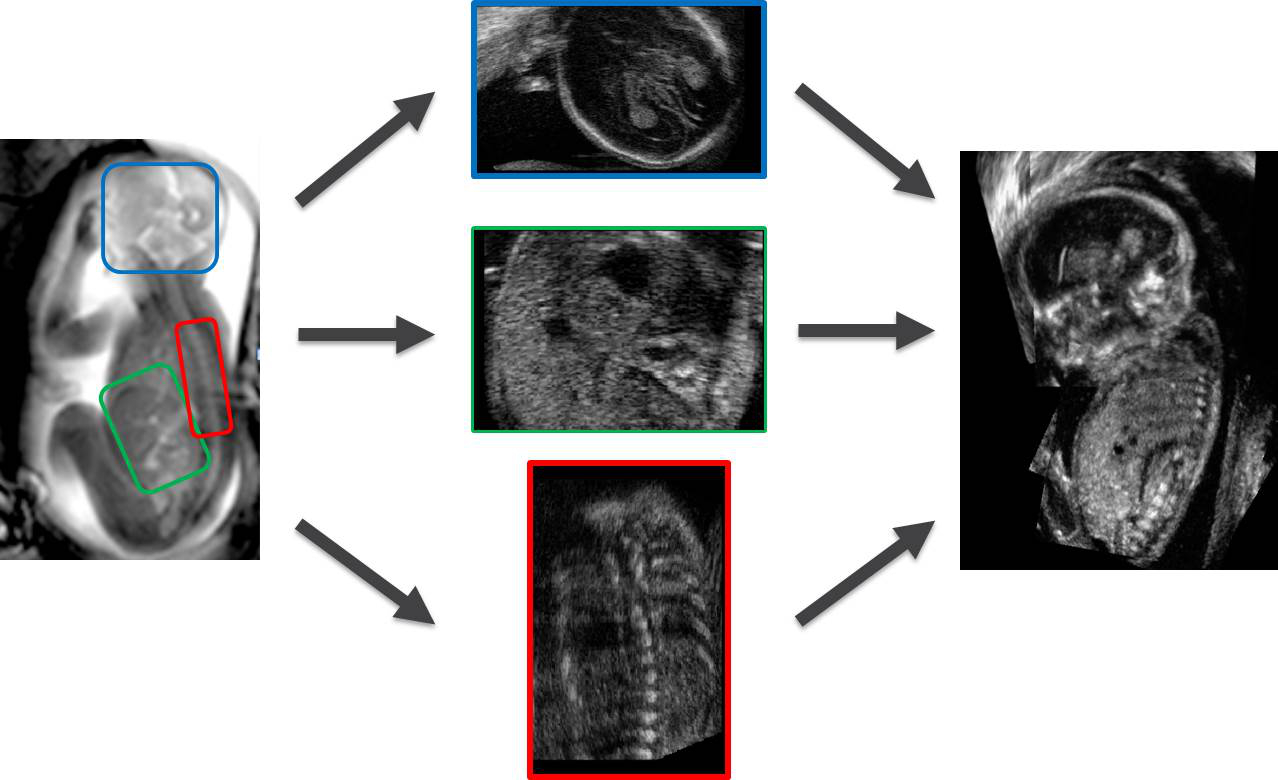
Figure 3: MRI images (far left) can be used as a template onto which comprehensive US data can (middle) can be mapped, to create a detailed fetal ultrasound-MRI atlas.
Computer Aided Diagnosis
Large datasets combined with statistical machine learning strategies can be used to extract information and learn patterns. This big data approach is having a massive impact in virtually every area of modern life, including computer vision, human-computer interaction, social media and healthcare.
- Use machine learning to automatically identify anatomical structures and potentially classify these as normal and abnormal.
- Develop Computer Assisted Diagnostics (CAD) to interpret ultrasound images.
Aim 4: Collect the clinical data and provide clinical evaluation and health economic evaluation to the project as well as exploring the business development opportunities
Our clinical teams from the Fetal Medicine Unit, Fetal Cardiology Unit and the Division of Women’s Health at St Thomas’ Hospital will work closely with the engineering teams to:
- Apply and evaluate the new fetal MRI and ultrasound techniques as they are developed with close feedback loops to the engineering teams.
- Collect high quality comprehensive datasets with both ultrasound and MRI for the building of the fetal atlas.
- Collect and annotate 20,000 fetal ultrasound image sets to build a database to mine for machine learning.
- Test the prototype system as a whole and evaluate its Computer Assisted Diagnostic abilities for clinical impact.
- Use the clinical results to perform a health economic evaluation and work with Philips to make a detailed case for developing the prototype into a clinical product.
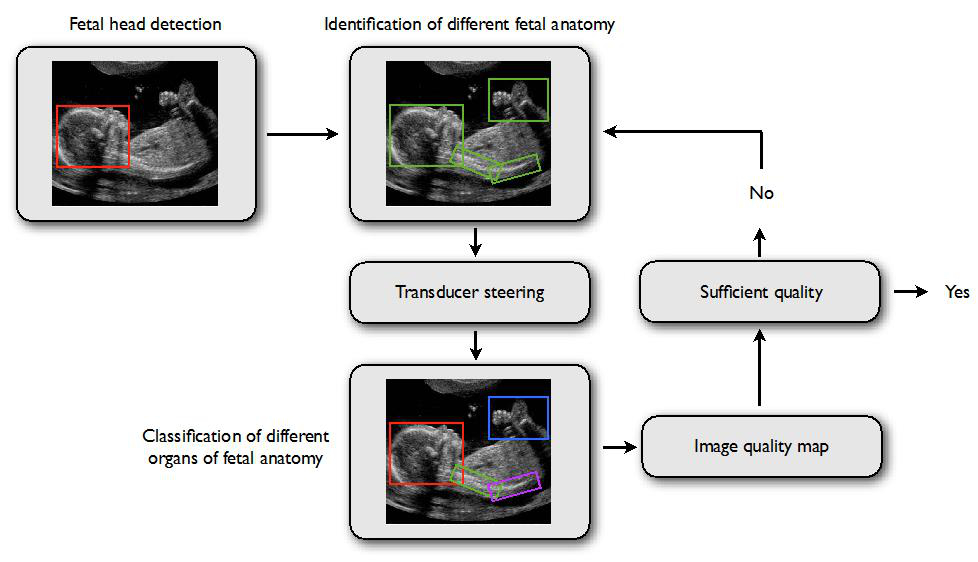
Figure 4: Ultrasound images collected will be annotated by the clinical team to enable organ detection. Fetal anatomy identification together with data on transducer position and ‘learnt’ measures of image quality will be used to produce ‘Image Quality Maps’- a measure of whether or not the ultrasound images acquired for a particular organ are of sufficient quality to allow a diagnosis to be made.
